This question may appear silly, since the very definition of a semiconductor is based on this property.
However, all but monocrystalline samples have the mobility gap rather than an energy gap.
Meaning, amorphous or powder like samples can mimic properties of a semiconductor with a proper energy gap even though they actually have no gap between bands whatsoever.
The reason is that, in theory, an energy gap exists only for periodic potentials extending over many periods (from $-\infty$ to $\infty$ in the Kronig-Penney model). Polycristalline or amorphous materials do not fulfill that requirement. Instead, they introduce various levels of disorder to the potential providing grounds for, so called, Anderson localization of states.
Due to disorder in potential within the material, many localized states would form.
However, despite the fact that Bloch’s theorem cannot be applied, there still be spatially extended states that would form a conductive region in energy diagrams
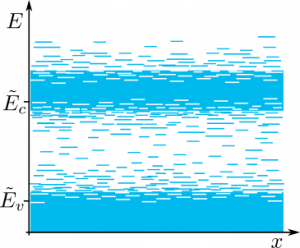
The region between values $\tilde E_v$ and $\tilde E_c$ is called mobility gap and consists of trap states that do not conduct current. Energy borders of the mobility gap, i.e., $\tilde E_v$ and $\tilde E_c$, are called mobility edges.